In the era of semaglutide and tirzepatide, a new, powerful agent has entered the research arena: Cagrilintide. But this isn’t just another GLP-1 agonist; it targets a completely different pathway to achieve one of the most profound appetite-suppressing effects ever observed in clinical trials. This article dissects the science behind this long-acting amylin analogue and explores its potential application for body recomposition.
Important Note: Cagrilintide is currently an investigational compound in clinical trials and is not approved by any regulatory agency for medical or recreational use. This article is for educational purposes only.
What is Cagrilintide? Understanding the Amylin Class
Cagrilintide is a long-acting synthetic analogue of amylin, a hormone naturally co-secreted with insulin by the pancreas in response to food intake. To understand its significance, we need to trace the evolution of amylin-based therapies.
Native Amylin: In your body, amylin works alongside insulin to regulate blood glucose and promote feelings of fullness after eating. It’s one of your body’s natural “stop eating” signals.
Pramlintide (Symlin): This was the first-generation synthetic amylin analogue, used as an adjunct treatment for diabetes. While effective, it required multiple daily injections and had a short duration of action.
Cagrilintide: Developed by Novo Nordisk, this next-generation analogue is engineered for once-weekly administration, making it much more practical for chronic weight management. Crucially, it belongs to a completely different drug class than popular GLP-1 medications like semaglutide.
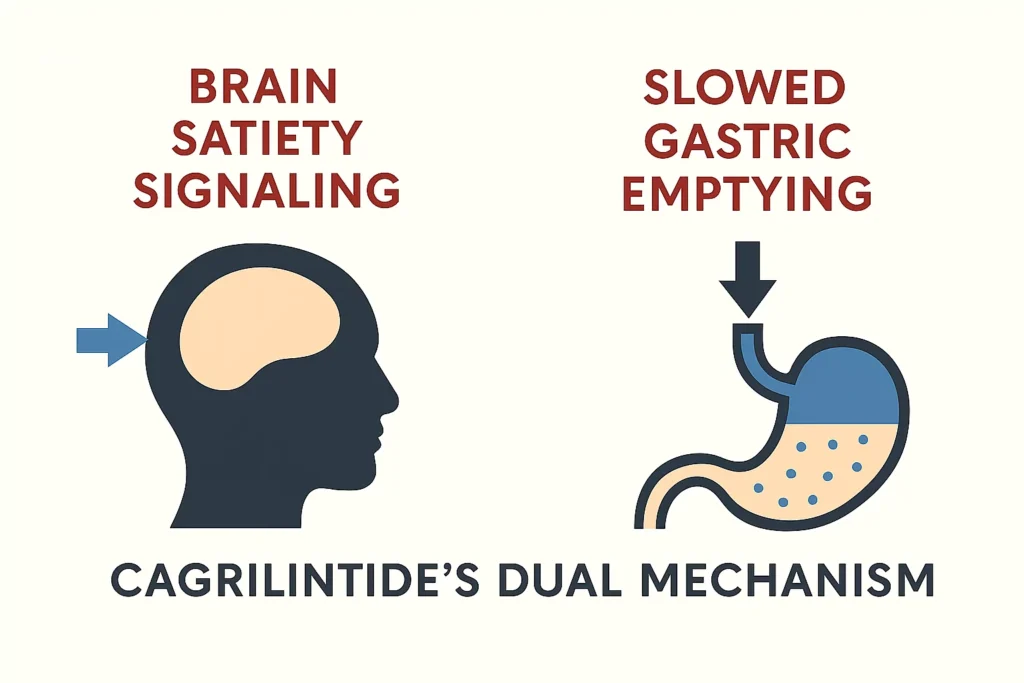
The Dual-Action Mechanism: How Cagrilintide Silences Hunger
What makes Cagrilintide so effective is its ability to suppress appetite through two distinct but complementary pathways.
The “Fullness” Signal in the Brain (Central Satiety)
Cagrilintide crosses the blood-brain barrier and activates amylin receptors in the hindbrain, specifically in the area postrema. This region acts as your brain’s “satiety center.” By stimulating these receptors, Cagrilintide directly signals that you’re full, significantly reducing hunger signals and the desire to eat.
Think of it as a “brain blocker” that directly interferes with the neurological commands that drive hunger.
The “Gut Brake” (Peripheral Gastric Effects)
Simultaneously, Cagrilintide slows gastric emptying—the rate at which food leaves your stomach and enters the small intestine. This prolongs the physical sensation of stomach fullness and distension after meals.
The practical effect: You feel physically full for longer periods, and the rapid return of hunger that often occurs 1-2 hours after eating is significantly delayed. This dual central and peripheral action creates a powerful, multi-layered defense against overeating.
The Evidence: What Clinical Trials Reveal About Its Potency
Phase 2 clinical trials have demonstrated Cagrilintide’s significant potential. A 2021 study published in The Lancet showed dose-dependent weight loss over 26 weeks, with the highest dose group achieving substantial reductions in body weight.
Perhaps even more impressive is the development of CagriSema—a combination of Cagrilintide with semaglutide. Early trial data suggests this multi-hormone approach may represent the next leap forward in weight management pharmacotherapy, potentially offering superior efficacy by targeting both amylin and GLP-1 pathways simultaneously.
Research Insight: Unlike compounds that work indirectly on fat metabolism like L-Carnitine, Cagrilintide directly targets the neurological and physiological drivers of hunger, making it potentially more effective for pure appetite control.
Cagrilintide for Bodybuilders & Athletes: A Cutting-Phase Power Tool?
Precision Dieting
Could make severe caloric deficits more manageable by eliminating cravings and reducing the psychological strain of dieting.
Lean Mass Preservation
By enabling more consistent diet adherence, it may indirectly help preserve muscle mass during aggressive cuts.
“Food Noise” Reduction
May quiet constant thoughts about food during cutting phases, allowing better focus on training and recovery.
However, it’s crucial to understand that this is different from peptides focused on recovery like those discussed in our guide to Why Athletes Are Turning to Peptides for Injury Recovery. Cagrilintide is purely metabolic in its action.
Cagrilintide vs. The Competition: A Comparative Analysis
| Feature | Cagrilintide | Semaglutide (Wegovy) | AOD 9604 |
|---|---|---|---|
| Primary Mechanism | Amylin receptor agonist | GLP-1 receptor agonist | Growth hormone fragment |
| Appetite Suppression | Direct brain signaling + slowed digestion | Mainly slowed digestion + mild brain effects | Minimal direct effect |
| Primary Use Case | Weight management | Weight management, diabetes | Stubborn fat loss |
| Dosing Frequency | Once-weekly (investigational) | Once-weekly | Daily |
| Legal Status | Investigational | FDA-approved | Research chemical |
For those researching other experimental compounds, our analysis of AOD 9604 Peptide provides context on another promising but different approach to body composition.
Potential Side Effects & Considerations for Athletes
Common Gastrointestinal Side Effects:
- Nausea and vomiting (most common, especially during dose escalation)
- Diarrhea or constipation from altered digestive timing
- Abdominal discomfort and bloating
The Practical Challenge: Nutrient Timing & Training
For athletes, the slowed gastric emptying presents a significant practical challenge. Timing pre-workout nutrition becomes difficult when your stomach empties slowly. A workout shake or meal might still be sitting in your stomach during training, potentially causing discomfort.
There’s also the risk of inadequate protein and calorie intake due to profound appetite suppression. Unlike traditional cutting phases where you might force-feed to hit protein targets, Cagrilintide could make consuming sufficient nutrients genuinely challenging.
Critical Consideration: The effects of Cagrilintide on metabolically healthy, highly active individuals are largely unknown. Most clinical data comes from obese or overweight populations with different metabolic profiles than trained athletes.
The Future & Current Reality
As of late 2023, Cagrilintide remains in Phase 3 clinical trials. Regulatory approval, if successful, is likely still years away. The most promising development appears to be the CagriSema combination, which ongoing trials are evaluating for both diabetes and obesity treatment.
Conclusion: A Powerful Tool in Theory, A Waiting Game in Practice
Cagrilintide represents a significant evolution in metabolic pharmacotherapy, moving beyond single-hormone targeting to sophisticated multi-hormone approaches. Its unique, dual-mechanism action on appetite regulation makes it theoretically a revolutionary tool for athletes during cutting phases.
However, its practical application is hampered by significant unknowns—particularly regarding side effect management in healthy populations and the very real challenges of nutrient timing for performance. While it offers a fascinating glimpse into the future of metabolic science, Cagrilintide remains a compound to watch closely through the scientific literature rather than seek out prematurely.
The most powerful tools in body recomposition remain those we can use safely and effectively today: precise nutrition, periodized training, and proven recovery strategies. Cagrilintide may one day join that arsenal, but for now, it represents potential rather than practice.