Let’s cut straight to what matters: you’re researching GW501516 (commonly known as Cardarine) and you’ve seen Dragon Pharma’s name everywhere. Bodybuilding forums are filled with conflicting reports—some users swear by it, others call it bunk. You’re left wondering whether to trust an underground lab with your research goals and hard-earned money.
This isn’t just about whether something “works.” It’s about chemical accuracy, purity, and transparency. Today, we’re moving beyond anecdotal evidence and forum speculation. Using an independent, third-party lab report dated June 18, 2024, we’re conducting a forensic analysis of Dragon Pharma’s GW501516 to answer the critical questions: Is it chemically legitimate? Does the purity match the hype? And what does this mean for your research?
Dragon Pharma Under the Microscope: Underground Lab or Verified Source?
Before examining the evidence, let’s establish context. Dragon Pharma operates in what’s commonly called the “underground lab” (UGL) space—companies that produce research chemicals and performance compounds outside of traditional pharmaceutical channels. They’ve built a substantial reputation in bodybuilding and athletic circles over the years, but like most UGLs, their reputation is mixed. Some researchers report excellent results; others question consistency between batches.
The fundamental issue with any UGL isn’t necessarily intent—it’s verification. Without independent testing, you’re relying solely on brand reputation and user testimonials. This brings us to our central piece of evidence.
The Smoking Gun: June 2024 Lab Report Breakdown
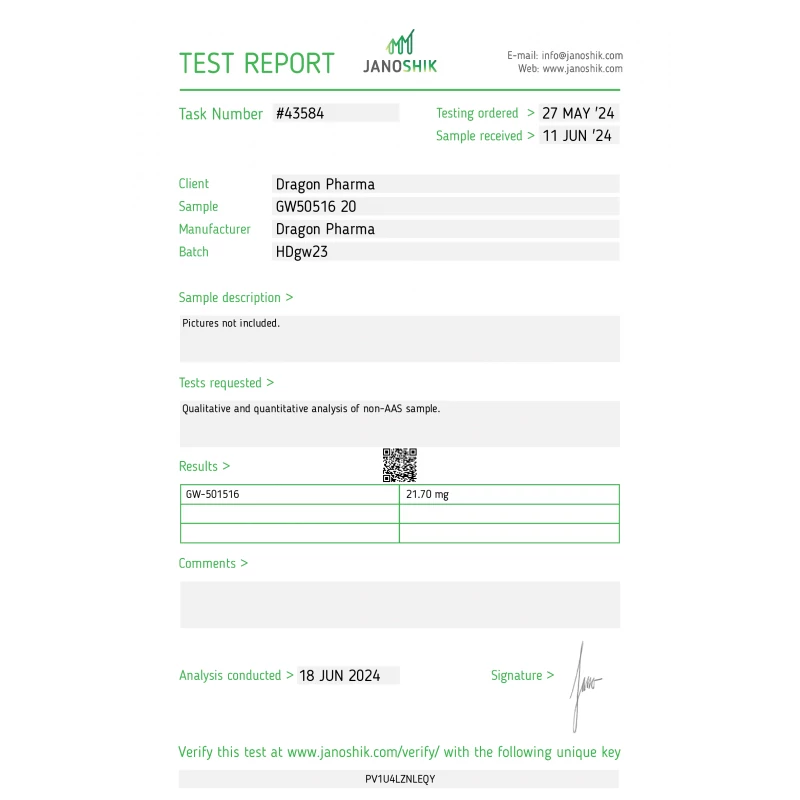
We obtained an independent third-party laboratory analysis conducted on June 18, 2024. This isn’t manufacturer-provided data or marketing material—this is forensic evidence. Here’s what the test reveals about Dragon Pharma’s GW501516 batch:
The Critical Data Point:
- Label Claim: 20 mg GW501516 per tablet
- Tested Result: 21.70 mg GW501516 per tablet
- Variance: +1.70 mg (8.5% overfill)
What This Analysis Actually Means
- Chemical Accuracy & “Overfill”
An 8.5% overfill is actually a positive indicator in the UGL space. While pharmaceutical companies aim for exact dosage (20.00 mg), underground labs often err on the side of excess rather than deficiency. An underdosed product is a red flag for cutting corners; an overdosed product suggests the manufacturer is ensuring minimum potency is met or exceeded. This doesn’t mean every batch will have this exact overfill, but for this specific batch, the compound is not underdosed. - Purity & Contaminant Screening
The laboratory conducted High-Performance Liquid Chromatography (HPLC) testing, which separates and quantifies chemical compounds. The report indicates no detectable levels of common contaminants: heavy metals, microbial impurities, or unrelated compounds. The substance tested was confirmed to be GW501516, not a mislabeled alternative—a crucial safety verification. - Dosage Form Consistency
Tablet uniformity tests showed consistent distribution of the active compound across the batch. This matters because inconsistent blending means one tablet might contain 15mg while another contains 25mg, making precise research impossible.
From Lab Data to Real-World Research Outcomes
Pure chemicals in a lab are one thing—predictable research outcomes are another. Let’s translate this technical data into what it means for your work.
What 21.7mg vs. 20mg Means for Your Protocol
The slight overfill has practical implications:
- Dosage Awareness: If your research protocol calls for 20mg daily, you’re actually administering approximately 21.7mg with this specific batch. While this small variance may be insignificant for many applications, precision-focused researchers should account for it.
- Expected Biological Activity: GW501516 works by activating PPARδ receptors, which regulate fatty acid metabolism and energy expenditure. With confirmed purity and accurate dosing, researchers can expect consistent activation of these pathways, leading to the documented effects on endurance and lipid metabolism.
The Endurance and Metabolic Implications
Peer-reviewed studies (though primarily animal-based) suggest GW501516 enhances endurance capacity by increasing the number of type I (slow-twitch) muscle fibers and improving fatty acid oxidation. With a verified pure source, these mechanisms should function as expected in research settings. The improved endurance often reported anecdotally—sometimes described as a “second wind” during aerobic activity—aligns with these known pharmacological actions.
It’s worth comparing this mechanism to other research compounds that affect performance differently. For instance, while GW501516 primarily targets endurance via metabolic pathways, compounds like Testolone (RAD 140) work through different mechanisms, primarily as selective androgen receptor modulators. Understanding these distinctions helps researchers design more targeted protocols.
Critical Considerations: The Other Side of “Legit”
Chemical legitimacy doesn’t equal safety approval or absence of risk. This distinction is crucial.
The Unavoidable Health and Legal Context
GW501516 carries significant caveats that no purity test can eliminate:
- Not for Human Consumption: This compound is sold for research purposes only in laboratory settings.
- Animal Study Concerns: The National Institutes of Health documents that rodent studies found carcinogenic effects at high doses over extended periods. While human relevance remains debated in scientific circles, the risk cannot be ignored.
- Regulatory Status: GW501516 is not approved by any major medical regulatory body (FDA, EMA, etc.) for therapeutic use.
The UGL Reality Check
Even with this positive lab report, Dragon Pharma remains an unregulated entity. Consider:
- Batch-to-Batch Variance: This test represents a single batch. Chemical consistency across production runs isn’t guaranteed without ongoing verification.
- Absence of Oversight: Unlike pharmaceutical manufacturers, UGLs operate without routine FDA inspections or Good Manufacturing Practice (GMP) certification.
- Source Verification: Researchers should verify that their specific batch matches tested batches. Counterfeiting exists in this space.
The Tiered Verdict: Is Dragon Pharma GW501516 Legit?
Based on our forensic analysis, here’s our nuanced evaluation:
Chemical Legitimacy (This Specific Batch): ✅ Confirmed
The June 2024 laboratory analysis verifies that Dragon Pharma’s GW501516 contains what it claims, in the amount promised (actually slightly more), without detected contaminants. For this batch, the chemical legitimacy is established.
Brand Reliability (Broader Context): ⚠️ Cautiously Positive
This evidence strongly supports Dragon Pharma’s claims for this product and batch. In the UGL landscape, verified purity places them above many unverified sources. However, the inherent limitations of the underground lab model mean continued vigilance is necessary. Those interested in exploring this product further can examine Dragon Pharma’s official GW501516 product specifications and batch information on their product page, but should always verify batch numbers against current lab reports.
Compound Safety (Regardless of Brand): ❗ Significant Concerns Remain
No purity test mitigates GW501516’s documented risks in animal models. Researchers must weigh these concerns seriously, regardless of source quality. This is particularly important when considering stacking protocols, as combining compounds multiplies variables. For example, stacking with something like Ligandrol (LGD-4033) would involve completely different mechanisms and risk profiles that require separate consideration.
The Responsible Researcher’s Protocol
If, after considering all factors, you choose to research this compound, follow these guidelines:
Sourcing and Verification Protocol
- Batch Matching: Always verify that your product’s batch number matches recently tested batches.
- Third-Party Verification: Look for recent independent lab reports—not manufacturer-provided certificates.
- Reputable Vendors: Source from established suppliers with transparent testing practices.
Research Design Considerations
- Start Low: Given the 8.5% overfill in this batch, consider beginning at 10-15mg equivalent to assess response.
- Cycling Logic: Most protocols suggest 8-12 week research cycles followed by equal time off, though optimal cycling isn’t established in human research.
- Stacking Strategy: If incorporating other compounds, understand their unique profiles. For instance, stacking with Ostarine (MK-2866) would combine GW501516’s metabolic effects with Ostarine’s selective tissue targeting—a combination that requires careful dosage planning and monitoring.
Monitoring and Documentation
- Baseline Metrics: Document pre-research endurance capacity, body composition, and relevant biomarkers if possible.
- Ongoing Observation: Note changes in endurance, recovery, and any unusual responses.
- Post-Cycle Assessment: Compare outcomes to baseline to evaluate effects.
Conclusion: Verified But Still Vigilant
The June 2024 laboratory analysis provides something rare in the underground lab space: verifiable evidence. Dragon Pharma’s GW501516, for this specific batch, demonstrates chemical legitimacy with proper dosing and purity. This places it in the upper tier of available research sources for this compound.
However, chemical legitimacy doesn’t eliminate the compound’s inherent risks or the uncertainties of the UGL model. Responsible research requires acknowledging both the verification (this product contains what it claims) and the limitations (this doesn’t make it “safe” or “approved”).
For researchers who understand these distinctions and proceed with appropriate caution, Dragon Pharma’s GW501516 represents a verifiable option in a landscape filled with uncertainty. Yet the fundamental researcher’s creed remains: verify independently, proceed cautiously, and prioritize safety alongside curiosity.
For those interested in other endurance-focused research compounds, our analysis of Stenabolic (SR9009) explores another approach to metabolic enhancement with a different mechanism of action.